Click here to see other posts about XPS
Only 10 $ for interpretation of each element in your XPS spectrum Payment Upon Completion Send your spectra...
What is XPS?
XPS is a surface-sensitive technique based on the photoelectric effect, which occurs when atoms or molecules are irradiated by photons of suitable energy, resulting in the ejection of electrons. The kinetic energy of the ejected electrons depends upon the elemental core level from which they originated.
Using this information, XPS data can be used to determine the elemental composition of the surface and, in most cases, the bonding environment of the elements.

The schematic figure shown to the right, illustrates the XPS procedure, where x-rays are used to excite and eject photoelectrons from a sodium chloride molecule on a substrate.
XPS detects and quantifies the ejected photoelectrons, which are proportional to the amount present in the uppermost layers of the surface.
To understand more about these uppermost layers, further details on the XPS penetration depth and attenuation length of the X-ray photons are required.
Normal XPS can provide information from the top 10nm (approximately) layer of the surface.
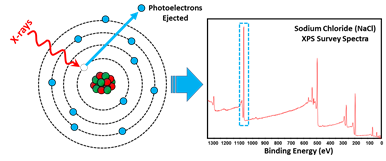 Schematic diagram of photoelectron emission from a sodium atom under x-ray exposure, and (right) example of XPS survey scan spectra of sodium chloride with the Na1s electron XPS peak highlighted in the spectra.
Schematic diagram of photoelectron emission from a sodium atom under x-ray exposure, and (right) example of XPS survey scan spectra of sodium chloride with the Na1s electron XPS peak highlighted in the spectra.
Image provided courtesy of Thermo Fisher Scientific.
Theoretically, XPS should be able to detect all elements. However, helium (He) does not readily form solid compounds and its 1s orbital has a tiny cross-section for photoemission.
Hydrogen (H) also has a tiny cross-section and suffers from having to share its only electron in forming compounds, which then resides in a valence-like orbital, the energy of which varies from compound to compound.
Features of the Thermo Fisher ESCALAB 250Xi XPS instrument
The Thermo Fisher Scientific ESCALAB 250Xi is the most recent advancement in the ESCALAB series.
The instrument is an optimised multi-method platform that is expandable and comes with excellent flexibility and configurability. Its cutting edge technology is driven by smart software and hardware.
Equipped with a micro-focusing X-ray monochromator designed to deliver optimum XPS performance, the instrument ensures maximum sample throughput.
The multi-technique capability and availability of a range of preparation chambers and devices provides the solution to any surface analytical problem.
 ESCALAB 250Xi XPS instrument
ESCALAB 250Xi XPS instrument ESCALAB standard sample loading chamber
ESCALAB standard sample loading chamber
Some notable features of the instrument include:
- High sensitivity spectroscopy
- Small area XPS
- Depth profiling capability with MAGCIS (Monatomic & Gas Cluster Ion Source)
- Ion scattering spectroscopy (ISS)
- Reflected electron energy loss spectroscopy (REELS)
- Micro-focused X-ray spot
- Efficient Charge Neutralisation
- Angle Resolved Spectroscopy
- Ultra-high sensitivity and energy resolution
XPS analysis sample requirement
XPS is a surface analytical technique that can be used to study the surface properties of a range of sample types. This includes both inorganic and organic (ex-situ and samples suitable to be placed under ultra-high vacuum) materials, polymers, semiconductors, metals, composite materials, geological and archaeological samples, ceramics, and glasses amongst others.
- The ideal dimension for an XPS analysis sample is 1cm X 1cm, with a maximum of 0.5cm thickness. Samples with lengths up to 5cm and widths up to 2.2cm can also be analysed. Samples below 0.5cm x 0.5cm dimension are difficult to mount on the sample stage. XPS analysis depends on the spot size. Ideally we can measure surface areas (spots) greater than 300µm X 300µm, as the smallest X-ray spot/exposure area is 200µm X 200µm for general analysis in our instrument. Please let us know your sample size and type in advance.
- Powder samples can also be measured using XPS. However, please discuss this with us in advance.
- Sample handling is crucial for XPS analysis. As XPS measures the top few atomic layers on the surface (different for depth profiling), it is very easy to contaminate samples with fingerprints. Ideally, XPS samples should be shipped and transferred inside a Wafer Carrier Box used in the semiconductor industry. However, any sealed container that doesn’t alter surface chemistry should be sufficient. Samples stored in zip-lock polymer bags are not ideal.

XPS Instrument Analysis Chamber view.
Image provided courtesy of Thermo Fisher Scientific.

Applications of XPS
XPS surface analysis can provide answers to a wide range of research problems. The following are examples of research questions addressed using XPS by researchers at the University of Brighton and elsewhere.
What material did we make / purchase?
XPS can provide data about the elemental distribution on the surface of a sample.
The limit of detection is 0.1 atomic per cent or better. In the figure (right), two XPS survey scans of gold coated QCM crystals are shown – the inset is a zoomed-in version of the Ti2p area of the spectra.
The two crystals are from two separate purchased batches. As can be seen, batch one (red) contains around 23 atomic per cent of titanium, making the crystal not fit for purpose, while batch two (green) is ‘pure’ as claimed by the manufacturer.
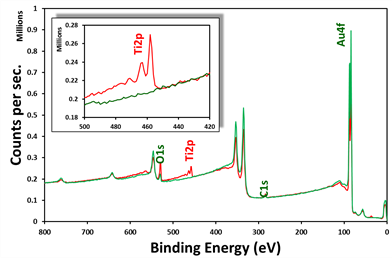
Survey scan spectra of two different batch QCM crystals. (S. Ray, unpublished data)
How thick is the coating/contamination on a surface?
XPS can measure precisely the thickness (below 10nm) of the surface adsorbed layer. This is useful in understanding the nature of surface contamination, and also in studying biomolecular adsorption on implants.
The spectra (right) is a comparison of XPS and Ellipsometric Thickness measurements of three different proteins adsorbed on hydrophobic surfaces, showing extremely close agreement.
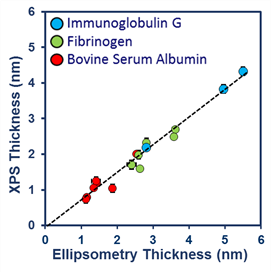 XPS and Ellipsometric Thickness measurement comparison of three different proteins adsorbed on hydrophobic surfaces.
XPS and Ellipsometric Thickness measurement comparison of three different proteins adsorbed on hydrophobic surfaces.
What chemical states are present on my surface?
XPS can identify and quantify the nature of the chemical states on a surface, and can help in visualising the surface functionalisation, essential for applications including bio-chemical sensors and chemical conjugations.
In the example (below left image), the experimental verification of graphene oxide reduction is shown, while the right image illustrates the success of a pluoronics (triblock copolymer) coating on reduced graphene oxide.
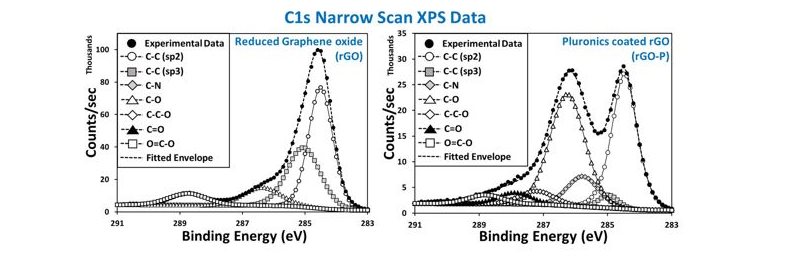 XPS C1s narrow scan spectra of (left) reduced graphene oxide and (right) pluronics coated reduced graphene oxide (S. Ray, unpublished data)
XPS C1s narrow scan spectra of (left) reduced graphene oxide and (right) pluronics coated reduced graphene oxide (S. Ray, unpublished data)
How can we analyse and quantify contamination or doping in our sample?
XPS can reveal surface or just below-surface contamination, and can successfully quantify any organic and inorganic contaminants and/or doping.
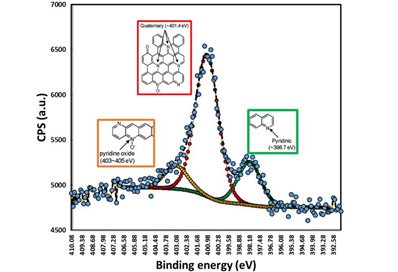
One example of doping quantification would be analysing the nitrogen doping effect on the properties of graphene. From the XPS spectra (right), the amount and bonding nature of nitrogen with graphene could be quantified.
Image on left: Nitrogen (N1s) narrow scan spectra and peak deconvolution to find the nature of nitrogen bonded to graphene (S. Ray, unpublished data).

Can XPS measure the thickness of coatings on nanoparticles?
In his 2017 publication, Professor David Castner (University of Washington) states, “Single particle information from electron microscopy combined with XPS sensitivity in determining composition make a powerful combination for nanoparticle analysis” ( Powell et al., 2017, J. Physical. Chemistry C).
XPS can measure precisely the thickness of single layer or multiple layers of coatings on nano-micro particles. Currently there are numerous situations where nanoparticles are used (e.g. in targeted drug delivery, sunscreens, and antimicrobial socks).
To functionalise these particles according to their target use, a proper understanding of the coating on the nanoparticles is required. In the case of multifunction nanoparticle use for targeted drug delivery, quantification of the single, double or triple layers is necessary.
XPS can help in understanding many other questions, including:
- What is the effect of heat, aging, chemical treatment, or real world use on my samples?
- What coating is on the surface?
- What is wrong inside my thick film?
- Are the thicknesses of film layers correct?
- Is the surface chemistry of a sample uniform?
Accessing the Surface Analysis Laboratory
If you have queries about how XPS can help in your research and/or industrial project, please do not hesitate to get in touch by telephone or email. We are happy to discuss your research requirements and provide quotations as necessary.
We particularly welcome proposals for joint research funding applications.
We also offer an analysis-only service, but can also provide full interpretation of results on request. XPS analysis costs can be charged per sample, per day/half-day or according to your needs.

